Key Takeaways
- Photoacoustic Microscopy (PAM) is a high-resolution imaging technique that integrates optical and acoustic methods to overcome the optical diffusion limit.
- It enables in vivo imaging at depths of several millimeters using endogenous and exogenous contrast agents.
- PAM is categorized into optical-resolution (OR-PAM) and acoustic-resolution (AR-PAM) types, each suited for different depth ranges. Applications include medical diagnostics, surgical guidance, and disease research.
- Avantier provides custom optics for PAM, supporting breakthroughs in imaging speed and quality, including real-time intraoperative histology.
Photoacoustic Microscopy
Photoacoustic microscopy (PAM) is a cutting-edge in vivo tissue imaging technique that combines optical and acoustic methods to break through the optical diffusion limit. It is capable of producing images with high spatial resolution at depths up to several millimeters and can simultaneously image multiple contrasts. One could, for instance, use these methods and different contrasts for anatomical, functional, flow dynamic, metabolic, and molecular image modalities.
How Does Photoacoustic Microscopy Work?
Photoacoustic microscopy begins with light: typically, a nano-second pulsed laser beam. It is this laser pulse energy that triggers the acoustic effect. Photons, absorbed by tissue, cause a local temperature rise. Weak acoustic scattering occurs as the tissues expand in a thermo-elastic way, and the resulting wide-band acoustic wave can be detected by ultrasound technology.
The energy is converted into a voltage signal, and a one-dimensional, depth-encoded image, called an A-line, is created for each laser pulse. 2D raster scanning is then used to form a 3D photoacoustic image from multiple one-dimensional A-lines.
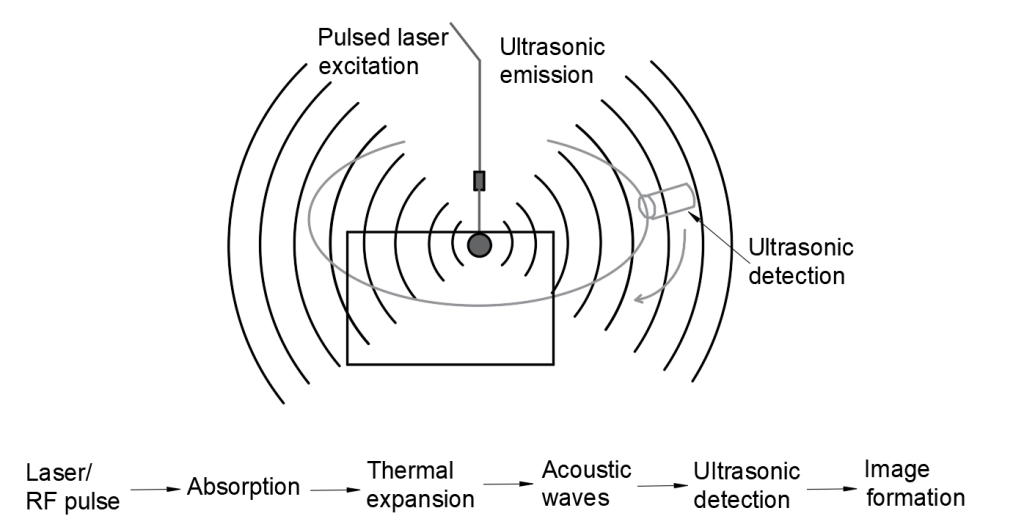
The key to in vivo imaging with PAM is the deep penetration of diffused photons and the low scattering of sound — 1000 times less than that of light. This enables researchers to produce quality imaging at a depth impossible with only optical methods and also enables them to determine the exact penetration depth at which imaging should occur. This versatility means one can use the same methodology for high resolution imaging of a mouse ear and a mouse brain, for instance. Axial resolution is determined by the bandwidth of the ultrasound transducer.
There are two main types of photoacoustic microscopy; OR-PAM, optical resolution photoacoustic microscopy, where the optical focus is tighter than the acoustic focus, and AR-PAM, acoustic resolution photoacoustic microscopy, where the acoustic focus is tighter. OR-PAM is typically used for depths up to 1 mm, and AR-PAM for depths from 1-3mm.
Optical properties of the tissue being imaged determine the contrast of the image. Non-invasive endogenous contrast agents such as intrinsic red blood cells, DNA, lipids, and glucose can be used as contrast absorbers. For other applications, exogenous contrast agents such as organic dyes, nanoparticles, or fluorescent proteins may be used.
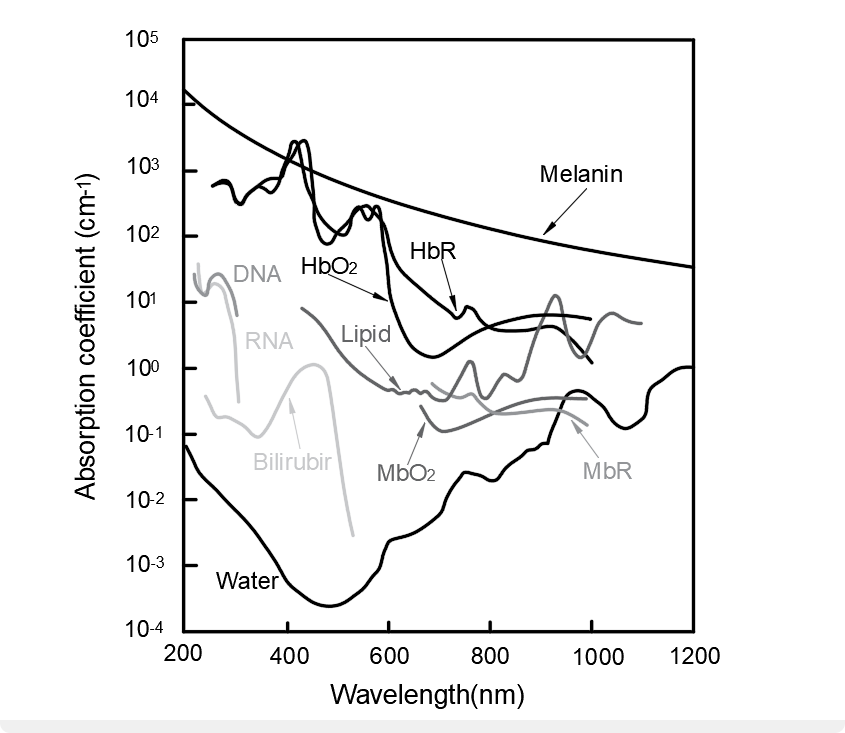
Applications of Photoacoustic Microscopy
PAM imaging is particularly important in medicine and research, where it provides a window into what is happening inside otherwise opaque tissue. It may be used for diagnostics, providing valuable data on blood flow, oxygen metabolic rates, or tumor growth. It can also be used to guide a surgeon’s knife in delicate surgeries, or to gain a better understanding of the changes that occur in diseased tissue.
One example of photoacoustic microscopy in action in medical research is in the study of inflammatory skin diseases. Using PAM combined with optical coherence tomography, researchers were able to determine oxygenation differences as well as thickened epidermis, vascular patterns with dilated vessels in a disorderly network, and the absence of melanin in eczematic in skin tissue.
Photoacoustic Microscopy at Avantier
At Avantier, we specialize in producing high quality custom optics for applications like photoacoustic microscopy. Our clients have been able to push past the limits of known technologies and achieve novel imaging speeds and quality. One example is the authors of a recent paper in Science Advances, Optical-resolution parallel ultraviolet photoacoustic microscopy for slide-free histology, who used a custom F-theta lens produced by Avantier to achieve the imaging speeds needed for potential real time intraoperative photoacoustic histology.
Do you have an optical project you’d like to take to the next level? Our experienced engineering and design teams are available to work with you to design and bring to production the exact optical components or systems your application requires. Contact us today for your next project.
References
- Attia ABE, Balasundaram G, Moothanchery M, Dinish US, Bi R, Ntziachristos V, Olivo M. A review of clinical photoacoustic imaging: Current and future trends. Photoacoustics. 2019 Nov 7;16:100144
- Ning, B., Sun, N., Cao, R. et al. Ultrasound-aided Multi-parametric Photoacoustic Microscopy of the Mouse Brain. Sci Rep 5, 18775 (2016).
- Rui Cao et al. ,Optical-resolution parallel ultraviolet photoacoustic microscopy for slide-free histology.Sci. Adv.10,eado0518(2024).
- Yao J, Wang LV. Photoacoustic Microscopy. Laser Photon Rev. 2013 Sep 1;7(5):10.1002/lpor.201200060. doi: 10.1002/lpor.201200060. PMID: 24416085; PMCID: PMC3887369.
- Zabihian B., Weingast J., Liu M., Zhang E., Beard P., Pehamberger H., Drexler W., Hermann B. In vivo dual-modality photoacoustic and optical coherence tomography imaging of human dermatological pathologies. Biomed. Opt. Express. 2015;6(9):3163–3178. doi: 10.1364/BOE.6.003163.
GREAT ARTICLE!
Share this article to gain insights from your connections!